Glucagon
As a counterregulatory hormone for insulin, glucagon plays a critical role in maintaining glucose homeostasis in vivo in both animals and humans. To increase blood glucose, glucagon promotes hepatic glucose output by increasing glycogenolysis and gluconeogenesis and by decreasing glycogenesis and glycolysis in a concerted fashion via multiple mechanisms[1].
Detailed quantitative studies assessing the cell composition of human islets are sparse [2–4]. These studies have reported that islets are composed of ≈70% β cells, 20% α cells, <10% δ cells, and <5% PP cells[5].
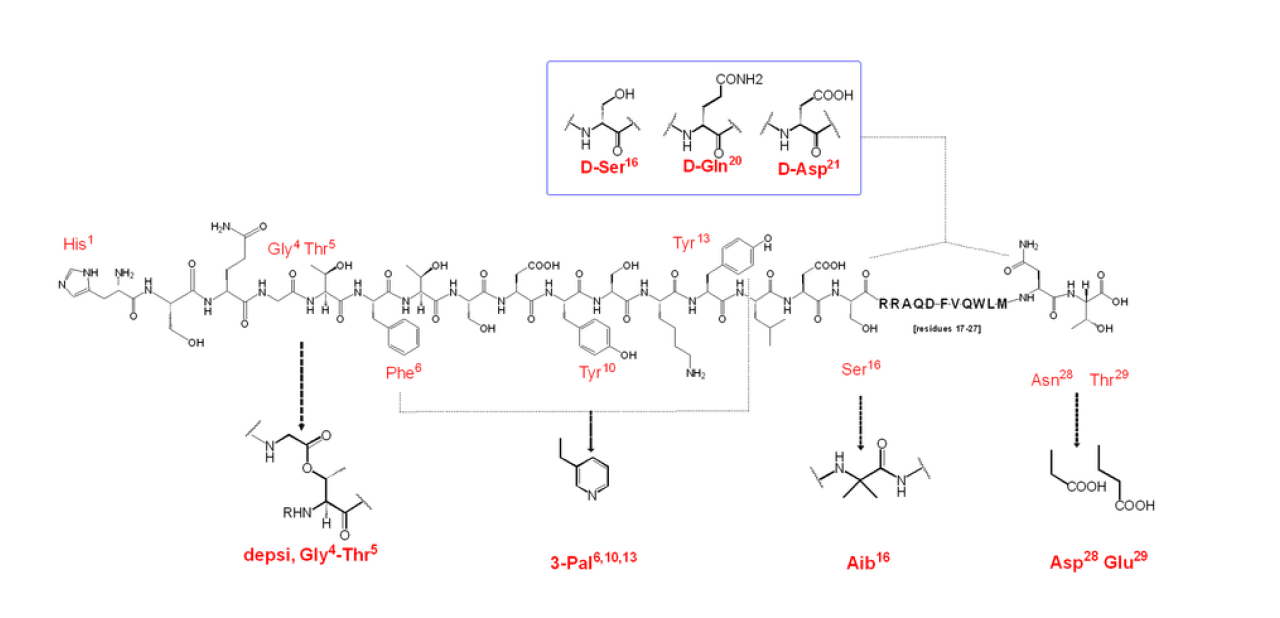
Proglucagon, a straight-chain peptide composed of 160 amino acids, is synthesized by intestinal L-cells, pancreatic islet a-cells, gastric a-cells and in certain neurons in the nucleus of the solitary tract in the brain stem [6–8]. In pancreatic a-cells, glucagon (1–29) is produced through processing of proglucagon by prohormone convertase 2 [9], which is molecular weight 3485 Da[10].The N-terminal histidine is a free amine and the C-terminus is a carboxylic acid[11].
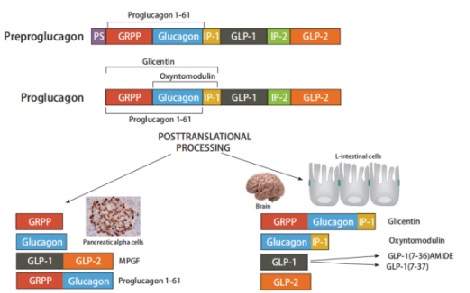
In the gastrointestinal tract, in contrast, glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), glicentin and oxyntomodulin are derived from proglucagon processed by prohormone convertase 1[12,13].
Glucagon acts via a seventransmembrane G protein-coupled receptor consisting of 485 amino acids [14],which has a half- life of 6 minutes[15].
Glucagon receptor gene (GCGR) encodes the receptor and has an abundant expression in liver and kidney and less expression in the heart, adipocytes, lymphoblasts, spleen, pancreas, brain, retina, adrenal gland and the gastrointestinal tract[16].
Glucagon secretion is regulated by insulin and somatostatin, (as the main paracrine/endocrine inhibitors), glucose, glucagon-like peptide-1 (GLP-1), amylin, leptin, fatty acids, ketone bodies—all inhibiting glucagon secretion, glucose-dependent insulinotropic peptide (GIP), amino-acids (as l-arginine, leucine)—stimulating glucagon secretion, and by the autonomic nervous system[17].
Contact Us
Follow us
 Wechat
Wechat

