ox-LDL
Atherosclerosis is a chronic, progressive disease which eventually leads to coronary heart disease (CHD), ischemic stroke and other atherosclerotic cardiovascular disease (ASCVD). Numerous studies have demonstrated an atherogenic role of oxidized lowdensity lipoprotein (ox-LDL) in the progression of ASCVD[1].
Oxidized LDL (ox-LDL) is the major modified form of native LDL because LDL particles are extremely sensitive to oxidative damage: each native LDL particle contains approximately 700 molecules of phospholipids, 600 molecules of free cholesterol, 1600 molecules of cholesterol ester, 185 molecules of triglycerides, and an apolipoprotein B-100 (apoB-100) protein with 4536 amino acids[2].
The oxidation of native LDL is a complex process that can be divided into three stages. During the initial stage, known as the lag phase, endogenous antioxidants such as vitamin E are consumed. During the proliferation phase, polyunsaturated fatty acids (PUFAs) in the lipids of the LDL particles can be rapidly oxidized to fatty acid fragments, oxidized phospholipids (ox-PL), and oxygen free radicals. During the decomposition stage, fatty acid fragments are converted to aldehyde, which can interact with the lysine residue of apoB-100 to form new epitopes. Importantly, these new epitopes inhibit the ability of LDL to bind to the LDL-Rs expressed on macrophages[3].
The most important atherogenic effect of ox-LDL is that this modification of native LDL shifts the recognition and internalization of the lipoprotein from the LDLRs to novel receptors termed as scavenger receptors (SRs).SRs are the cell surface receptors expressed on macrophages and other vascular cells that recognize and internalize ox-LDL rather than native LDL; these include SR-A, cluster differentiating 36 (CD36), SR-BI, cluster differentiating 68 (CD68), scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX), and lectin-like oxidized LDL receptor-1 (LOX-1), among others[5]. Among these, SR-A binding to oxidized lysine residue of apoB-100 and CD36 binding to ox-PL are responsible for most of the ox-LDL uptake by macrophages in vitro[6,7]. The most important point is that contrary to LDL-R, SRs are not down regulated by elevated levels of intracellular cholesterol [8].
Uptake of ox-LDL by macrophages through SRs leads to remarkable cholesterol accumulation, converting macrophages to foam cells and promoting the development of atherosclerotic lesions[9].
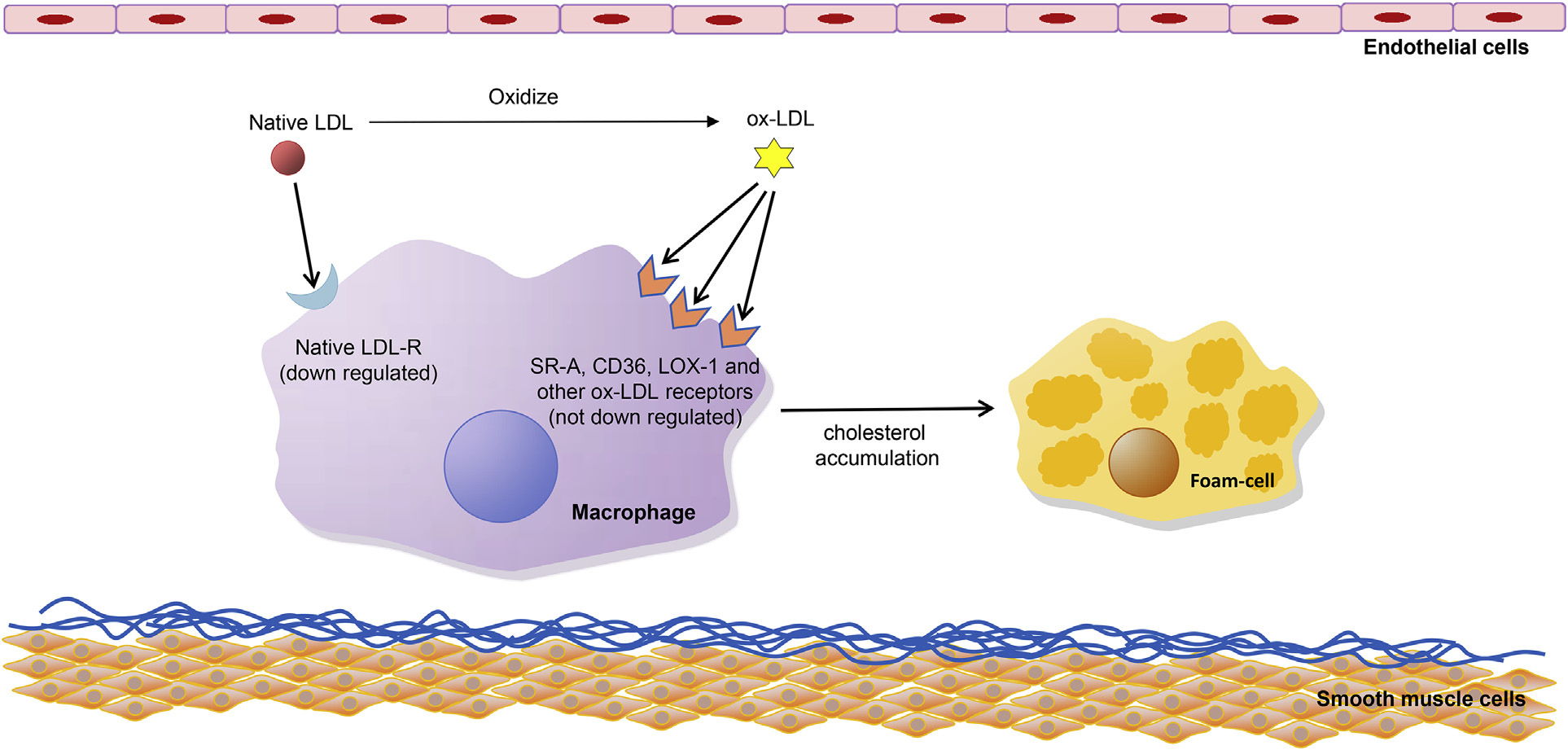
Mechanisms of ox-LDL uptake by macrophages.
S.Gao, J. Liu / Chronic Diseases and Translational Medicine 3 (2017) 89e94
To date, three monoclonal antibodies have been developed for determination of circulating ox-LDL by enzyme linked immunosorbent assay (ELISA): ox-LDL-4E6[10],ox-LDL-E06[11],and ox-LDL-DLH3 antibody[12]. ox-LDL-4E6 was the first to be established and widely used. It can directly recognize the epitope that is generated after modification of lysine residues in apoB-100 by aldehydes, while DLH3 and E06 antibody recognize oxidized phosphatidylcholine (ox-PC) generated after modification of phospholipids containing PUFAs[13]. However, ox-LDL with fewer than 60 lysine modifications cannot be detected using the ox-LDL-4E6 antibody[13].

Contact Us
Follow us
 Wechat
Wechat

